What is it about?
Monkeypox is a zoonotic disease caused by a virus belonging to the Poxviridae family, the Chordopoxvirinae subfamily and the Orthopoxvirus genus. Poxviruses are large and brick-shaped with an oval double-stranded DNA and a diameter of 200-400 nm.
The World Health Organization reported in 2018 that monkeypox is a disease that mainly affects animals in the equatorial rainforests of western and central Africa. In 1970, during an epidemiological investigation in the Democratic Republic of Congo, some scholars found the first case of infection in a human. Its natural incubator is a striped squirrel that lives only in West and Central Africa. Furthermore, recent studies have reported that rats, striped mice, dormice and monkeys can also be infected. Studies on other potential incubators are still ongoing.
Effects on humans
Monkeypox is usually a self-limiting smallpox-like disease and involves a pathognomic lesion in the lymph nodes in the form of lymphadenopathy and swelling. The incubation period usually varies from 7-14 days (sometimes 5-21 days) after the initial exposure. The disease that progresses after infection is distinguished by two phases:
- The initial invasion period (within 0-5 days) is characterized by fever, headache, muscle aches, back pain, swollen lymph nodes, and fatigue.
- The second phase is characterized by the development of skin rashes such as macular, papular, vesicular, pustular crusts that gradually present themselves within 1-4 days from the onset of fever.
Their spread affects the area from the face to the trunk. Some studies also report other complications, including vomiting, diarrhea, corneal scarring, conjunctivitis, bronchopneumonia, sepsis, vision loss, encephalitis, and secondary microbial infections.
The most severe cases are commonly associated with children. The mortality rate among the unvaccinated population is historically recorded from 0 to 11%.
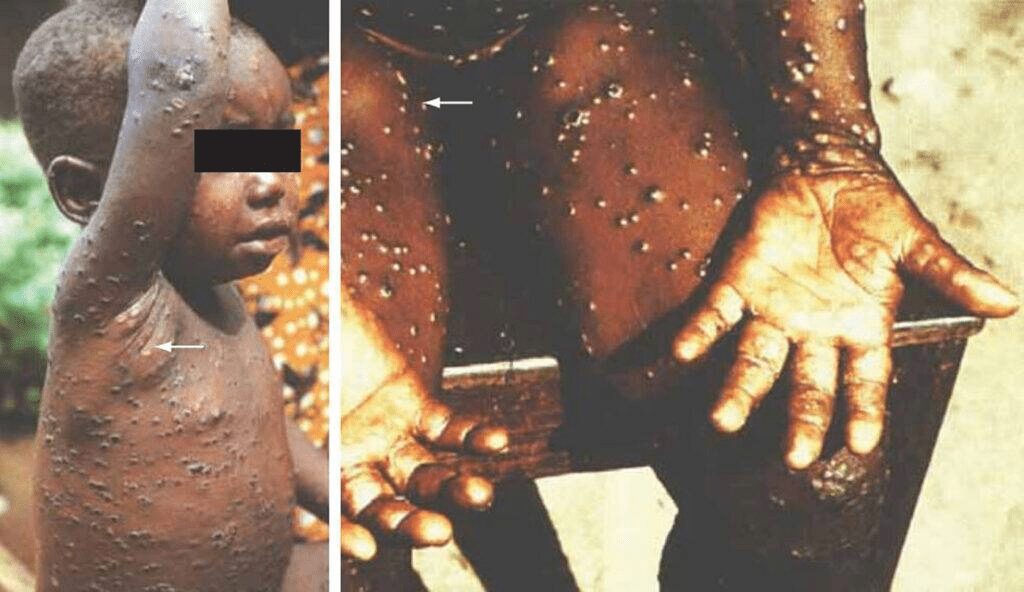
[Source: Parker S., et. al., 2007. Human Monkeyprox: an emerging zoonotic disease. Future Microbiology, 2(1), 17-34]
How is it transmitted?
Monkeypox infection can only be transmitted through direct or indirect contact with live and / or dead infected animals. Direct transmission includes bites or scratches from infected animals, contamination of raw meat, contact with body fluids. Indirect consists of contact with bedding, clothing or surfaces.
Human-to-human transmission has previously been reported via respiratory droplets and contact with bodily fluids, skin lesions from an infected individual and / or via a contaminated object, such as bedding contaminated with the patient’s bodily fluids.
A realistic scenario?
The concern of the health authorities had already proved high. On 23 July, the WHO declared that this is a new “global health emergency”. Several countries have an optimal environment to “hide” the spread of monkeypox by modeling ecological niches. This creates difficulties for standard surveillance levels. For example, in Ghana the disease circulates widely among animals, although there are no recorded cases of human infection. This raises concerns about the traceability of its circulation.
New studies
There are studies that reveal a good ability of poxviruses to adapt to immune defense mechanisms. These are capable of carrying out rapid episodes of transient gene amplification. In particular, the monkeypox virus contains in its genome many proteins which confer resistance to interferons: K3L (P18378) is a protein with homology to the eukaryotic initiation factor 2 (eLF-2alpha). The KL3 protein inhibits the action of PKR (protein kinase that activates interferons).

Consequently, the fundamental question is how poxviruses, despite their low mutation rate, efficiently explore the mutational space and overcome rapidly evolving immune factors, such as PKR, as they move between host species. Currently, new emerging cases have been detected in several non-endemic countries, therefore where the disease is not characteristic of the place or of a population.

Future prospects
The actual risk varies from region to region. In the European area, attention remains high, due to widespread outbreaks and atypical symptoms. It is the latter that are of greatest concern.
The atypical features described include: presence of few or a single lesion, absence of skin lesions (in sporadic cases), anal pain and bleeding, lesions in the genital or perineal / perianal area that do not spread further, lesions appearing at various stages (asynchronous) development, the appearance of lesions before the onset of fever, the absence of the prodromal period.
The Food and Drug Administration (FDA) has authorized the use of the antiviral agent Tecovirimat, also approved by the European Medicines Agency (EMA).
New studies
A recent study published in the Lancet reports the case of a mother who came into contact with smallpox through her infected daughter. Initially, the woman developed headache, malaise, pharyngitis and vesicles on the chest, then tested positive by PCR method. In this phase, the patient was isolated for 35 days in hospital and then underwent treatment with a 2-week course of oral Tecovirimat (600 mg twice daily).
Samples taken from blood and respiratory tract were negative for PCR amplification 48 hours after treatment. No lesions developed after 24 hours. In addition, the haematological, renal and hepatic profiles remained unchanged.
On day 7 of the treatment the patient was discharged to continue the hospitalization at home. Furthermore, she was clinically healthy and afebrile during and after the end of therapy. Of particular note is that in parallel tests on other patients, two doses of Brincidofovir generally reduced the viral load. However, the limit of the study is the use of a small sample (only seven patients) and highlights a course of the disease that is expensive to manage, even in a high-income country like the United Kingdom.
Original article by Nicola Lovecchio – Translated by Umberto Lazzaro
Source:
- Adler, H., et. al., 2022. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet. 22(8): 1153-1162. DOI: https://doi.org/10.1016/S1473-3099(22)00228-6
- Alakunle, E., et. al., 2020. Monkeypox virus in Nigeria: Infection biology, epidemiology, and evolution. Viruses. 12:11. DOI: https://www.mdpi.com/1999-4915/12/11/1257#
- Elde, N.C., et. al., 2012. Poxviruses Deploy Genomic Accordions to Adapt Rapidly against Host Antiviral Defenses. and Cell. 150(4): 831-841. DOI: http://dx.doi.org/10.1016/j.cell.2012.05.049
- Jezek, Z., et. al., 1988a. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull Wolrd Health Organ. 66(4): and 459-464. PMCID: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc2491168/
- Nakazawa, Y., et. al., 2015. A Phylogeographic Investigation of African Monkeypox. Viruses. 7(4): 2168-2184. DOI: https://www.mdpi.com/1999-4915/7/4/2168/htm#
- Parker, S., et. al., 2007. Human Monkeypox: an emerging zoonotic disease. Future Microbiology. 2(1): 17-34. DOI: http://dx.doi.org/10.2217/17460913.2.1.17
- Vaughan, A., et. al., 2020. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerging Infectious Diseases. 26: 782-785. DOI: http://dx.doi.org/10.3201/eid2604.191164