Original article: Membrana plasmatica, by Vanessa Vitali
Every cell has a lipid and protein layer, called cell membrane (or cytoplasmic or plasma), which defines its boundaries and regulates molecular exchanges with the external environment. This structure can even be called the inner membrane to distinguish it from the outer membrane present in gram-negative bacteria. The plasma membrane encloses the cytoplasm, the nuclear region (nucleus for eukaryotic cells), and the organelles located inside the cell, necessary for its vital functions. Its composition – formed by lipids and proteins – allows it to be resistant yet flexible, self-sealing, and permeable to molecules selected from the cell itself. Besides this form of passive transport, specialized membrane proteins promote exchange through energy-intensive transport – also called active transport.
Structure of the cell membrane
The plasma membrane is composed (both for the eukaryotes and prokaryotes) of two types of molecules: phospholipids and proteins. The difference between the two cells consists of carbohydrates and sterols, which give the eukaryotic plasma membrane rigidity. Since it does not contain sterols, the prokaryotic plasma membrane is less rigid. However, there is an exception: the bacteria of the genus Mycoplasma have sterols in the membrane, thus achieving rigidity and compensating for the cell wall lack.
The thickness of the membrane can vary from 5 to 8 nm. The fluid movements of the phospholipid and protein molecules allowed the fluid mosaic model to be determined. This fluidity is due to the non-covalent interactions between the membrane components, that enable the molecules to move freely laterally in the membrane.
Phospholipids are arranged in a bilayer, in which the polar heads face outward and the non-polar tails face inward. This disposition is because the tails of the phospholipids are hydrophobic and so they “escape” from water. On the other hand, the polar heads are hydrophilic, and for this reason face outward to interact with the aqueous phase inside and outside the cell.
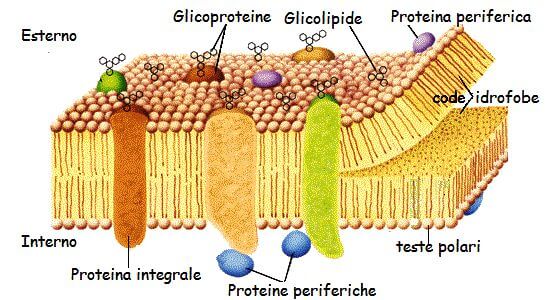
The membrane proteins are immersed between the phospholipids. They are kept in the right position thanks to the hydrophobic interaction between the membrane phospholipids and the hydrophobic domain of the proteins. Based on their disposition in the membrane, we can distinguish:
- peripheral proteins, like enzymes or supporting structure;
- integral proteins, like transport proteins.
The proteins carry out different essential tasks, like transporting molecules into or out of the cell, structural function, and enzymatic function.
Phospholipids
Phospholipids are membrane lipids, and as such, lend themselves to the construction of all biological membranes. They are mainly divided into two parts:
- Head: consisting of glycerol, phosphoric acid, and a very polar molecule (substituent group). The third carbon C of glycerol is bonded to the acid and the polar molecule through a phosphodiester bond: the phosphoric acid residue esterified to the alcohol group of the glycerol binds to the molecule. The substituent group varies according to the typology of the phospholipid, and it is a polar molecule, like choline, ethanolamine, etc. So the heads of the phospholipids are polar and hydrophilic (similar to water).
- Tails: constituted by two fatty acids, bonded through an ester bond to glycerol’s first and second C carbon atoms. The tails are apolar, hydrophobic (they run away from water). Fatty acids types varies depending on the type of phospholipids.
The differences in the heads and the tails determine the arrangement of phospholipids in the membrane construction. Placed in an aqueous solution, the phospholipids form a structure in which the tails (since they are hydrophobic) are organized inward, away from the water. As a result, the hydrophilic heads contact the water and position themselves outward to close the structure. For this characteristic, phospholipids are considered amphipathic molecules.

Phospholipids in water can form different structures:
- Micelles: spherical arrangements, consisting of molecules – dozen to few hundred;
- Double layer: two layers of phospholipids make up the double-layer structure that we know, with the hydrophobic tails inward and the hydrophilic heads outward. Being an open structure, the sides are exposed to the water, forming an unstable system. Spontaneously, it creates a lipidic aggregate called a vesicle;
- Vesicles: also known as liposomes. These structures can incorporate water in an inner compartment, separated by the hydrophobic tails.

Phospholipids biosynthesis begins with acyl-coenzyme A acylation of a glycerol-3-phosphate molecule. Thus, phosphatidic acid is formed, the precursor to all phospholipids and from which the name is also derived.
Phospholipids can be degraded in an organism by the phospholipases’ hydrolytic enzymes, which catalyze the cleavage of ester bonds.
Membrane proteins
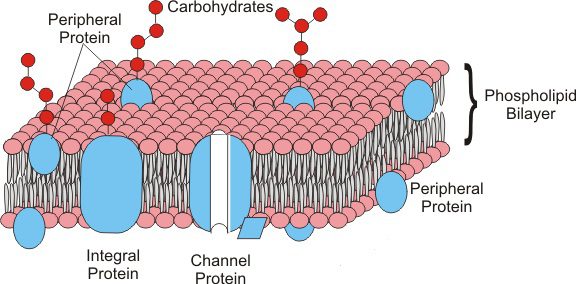
Membrane proteins are immersed between phospholipids in the fluid mosaic. The position of proteins in the membrane is maintained by hydrophobic interactions between the lipids and the hydrophobic domains of the proteins. This position can be peripheral or cover all the thickness of the membrane. Based on this disposition, the proteins are defined:
- Peripherical proteins: placed in the superficial part of the membrane, like enzymatic or structural proteins;
- Integral proteins: immersed in the membrane and, in some cases, go throught it from side to side (transmembrane proteins). Among these, we can find the channel proteins (pores) that form a passage channel that allows molecules to go in and out of the cell. An example are the transport proteins.
Amphipathic proteins are also important, placed both in the cytosol and in association with the membranes; their affinity for the membrane is due to a non-covalent binding to a membrane protein or lipid.
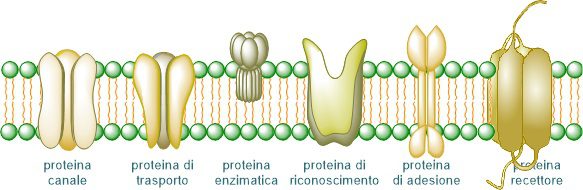
The main functions carry out by the membrane proteins are:
- Enzymatic: they catalyze chemical reactions that occur at the membrane level;
- Supporting or structural: they maintain the structure of the cell membrane;
- Transport: they allow the translocation of molecules to the inside or outside of the cell;
- Cellular interactions: they are involved in the communication between cells:
- Recognition: they have structures that allow the identification of molecules;
- Receptors: they have structures that allow them to receive and recognize signals, like hormones As a result, some cellular processes can be initiated.
Membrane proteins can be associated with carbohydrates (glycoproteins) or lipids (lipoproteins). Glycoproteins are abundant in the membrane and they expose their glucidic groups to the outside of the cell where, together with the glucidic groups of glycolipids, constitute a glucidic coating on the surface called the glycocalyx. Thanks to the formation of hydrogen bonds, the glucidic groups help to give stability to the plasma membrane. Besides this function, the glycoproteins are membrane receptors for hormones and growth factors. Moreover, they intervene in cellular interactions and are recognition sites for bacteria and viruses.
In bacteria, the lipoproteins play essential roles in physiology, and they are peripherally anchored to the plasma membrane. Thanks to the general secretion or Sec pathway, the proteins get a sequence modification in a cysteine residue and cleavage of a signal peptide by peptidases. These modified lipoproteins can be anchored to the plasma membrane. In bacteria their roles are:
- Providing membrane stability;
- Role in cell division, sporulation, conjugation;
- Acquisition of nutrients;
- Signal transduction;
- Transport;
- Protein folding;
- Mechanisms of pathogenicity: role in adhesion, colonization, invasion, persistence.
Functions of the plasma membrane
The primary function of the plasma membrane is containment since it contains all the cell contents, such as the cytoplasm and the organelles.
The membrane functions are also carried out by the proteins present inside; among these, there are:
- Extracellular communications with the adjacent cells through specific proteins;
- Identification of molecules thanks to proteins able to recognize their structure;
- Receive signals, like hormones helpful to determine the initiation of cellular processes, for example, the activation of the defense mechanism;
- Transport of substances.
The transport through the membrane can be passive or active. Let’s consider a bacterial cell placed in a hypertonic solution – a place in which the concentration of solutes is higher than the one present in the cell. The cell undergoes a reduction in volume and wrinkles because the water moves to the environment with a higher concentration of solutes (osmosis). If we put the cell in a hypotonic solution – a place in which the concentration of solutes is lower than the cell – the water will go into the cell, attracted by the higher concentration.
When osmosis is not possible, to move the molecules, the cell will use the active transport thanks to membrane proteins and expenditure of energy molecules (ATP).
Another form of transport used in this case is group transport, present only in the prokaryotic cells. It allows the accumulation of substances against a concentration gradient. This form of transport requests expenditure of energy and modifies the substance during the passage through the membrane. The latter becomes thus impermeable to the new substance, preventing its exit.
Particular translocation pathways across the plasma membrane
In bacteria exist two pathways of translocation across the cell membrane:
- A translocally coupled mechanism present in all organisms mediated by signal recognition particles;
- A bacterial specific mechanism mediated by the Sec and ATP complex.
Sec is a protein complex in bacteria like Escherichia coli, and its main component is the conduction protein channel present in the cell membrane. In bacteria, this channel is made by proteins called SecY, SecE e SecG. It is unknown the motivation of two translocation pathways yet, but it is thought it is due to a limitate number of SecYEG channels. According to studies, the channels present are insufficient to support translocation solely by the translocally coupled mechanism.
SecY forms an hourglass-shaped channel in the plasma membrane. At the side of the central narrowing of the hourglass are aliphatic long-chain residues. During translocation, substrate proteins cross the narrowing. In the resting state, however, the channel is closed with a helical alpha plug domain. SecE and SecG stabilize SecY: externally, but on both sides, SecE is bound to SecY, and in its absence, undergoes rapid degradation.
SecYEG can be bound in three forms:
- Close: the translocation doesn’t happen;
- Partially close: the bond between SecAEG and SecYEG in the presence of ADP helps a slight opening of the channel;
- Open: the link between SecAEG and SecYEG with ATP causes an expansion of the narrowing present in the channel.
In addition, SecA performs the translocation of most E. coli proteins. SecA has two domains: NBD1 and NBD2. The primary structure of NBD1 is interrupted by a polypeptide cross-linking, which tracks the substrate polypeptide that has to enter the cell. NBD2 is composed of the alpha-helical scaffold domain, which intercepts the substrate protein, and the alpha-helical wing domain.
The translocation through SecA takes place in two phases:
- Substrate proteins that need to be translocated are addressed into the Sec complex;
- Translocation by SecYEG.
Through interaction with the substrate protein or SecYEG, SecA changes its configuration: the polypeptide cross-link is rotated and gets closer to NBD2 to assume an open conformation. It then forms a cavity between NBD1, NBD2 and polypeptide cross-link, in which SecA binds to the substrate for translocation.
Bibliography
- http://www.treccani.it/enciclopedia/fosfolipidi/
- Lehninger, Nelson, Cox, I principi di biochimica di Lehninger, edizione 2011, Zanichelli Editore.
- https://www.sciencedirect.com/science/article/pii/S0167488914001402
- https://books.google.it/books?hl=it&lr=&id=g4i-LFx2oMIC&oi=fnd&pg=PR13&dq=flagelli+e+fimbrie&ots=viHp0FV5Y_&sig=FUMfPH_VwSmFJa2u3h9ZFJJVatI&redir_esc=y#v=onepage&q=flagelli%20e%20fimbrie&f=false
- http://testdimedicina.altervista.org/blog/componente-proteica-membrana-cellulare/
- https://www.gmpe.it/biologia/membrana-plasmatica
- https://www.medicinapertutti.it/argomento/glicoproteine-di-membrana/
Figures
- https://www.chimica-online.it/biologia/membrana-cellulare.htm
- https://commons.wikimedia.org/wiki/File:OSC_Microbio_07_03_micelle.jpg
- http://www.editricesanmarco.it/articoli/sfogliabili/101-4/files/assets/seo/page16.html
- https://www.sutori.com/story/la-cellula-procariote–qSDvEmz9GFuWYmsyS4egmiho
- https://www.tes.com/lessons/QToW1v9X0G4Fmg/la-cellula